Protocol Submission Guidelines
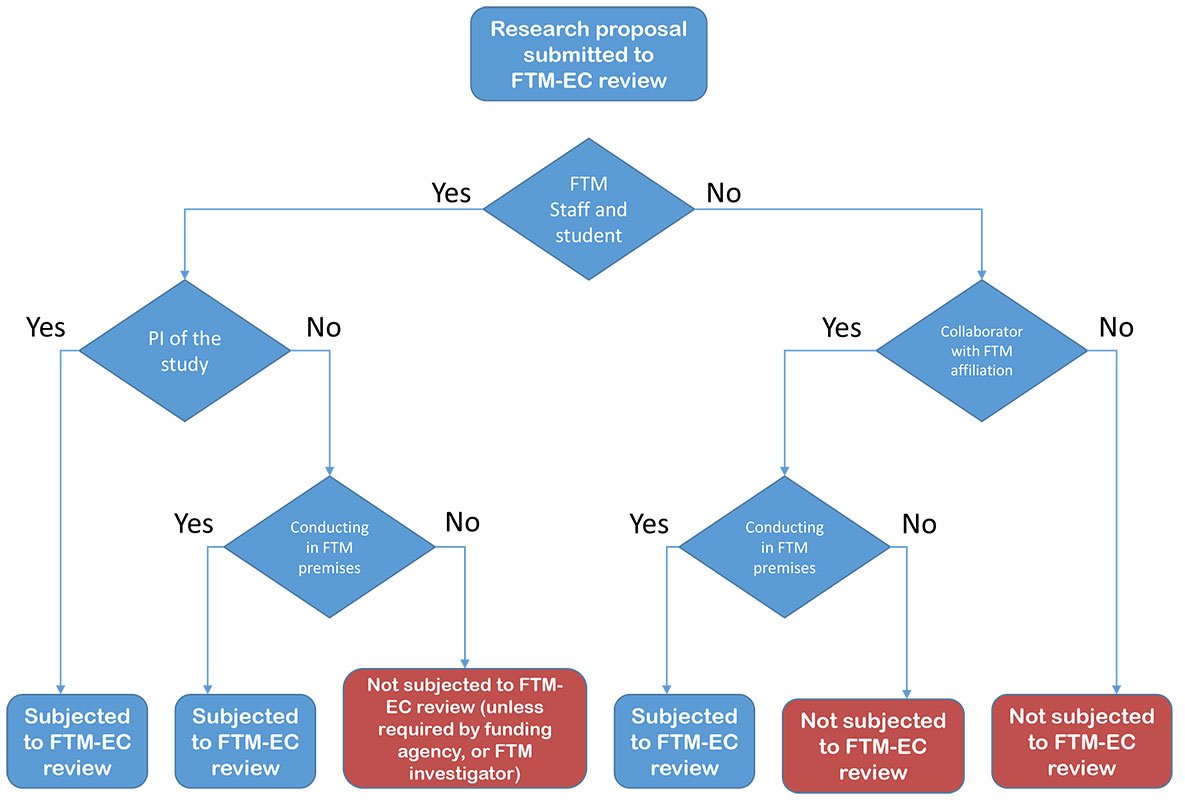
1. Research involving human subjects, which is subject to a review by the Ethics Committee, Faculty of Tropical Medicine, Mahidol University comprises:
- Research where FTM staff members/ students are Principal Investigator conducting their research within or outside FTM facilities. Where the research is conducted outside FTM facilities, the Principal Investigator must also submit the research to the local EC for consideration; or
- Conduct the research in FTM facilities with Investigator(s) affiliated with FTM
- Research conducted with clients of the Hospital for Tropical Diseases, Faculty of Tropical Medicine, Mahidol University
(Please note that in the case of thesis research, it is required that the supervisory committee has been established, and has approved the thesis proposal. The date of approval of the thesis proposal must be recorded on the ethics protocol cover sheet)
Click here to see policy and scope for review.
2. Ethics Review Fee / Submission Fee
Under the Regulation and Announcement of the Faculty of Tropical Medicine, dated 3 October 2022, research studies submitted for ethical review are subject to a fee, according to the following schedule:
| Initial Review | ||
| Type of Fund | Fee (Baht) | |
| 1 |
|
5,000 |
| 2 |
|
10,000 |
| 3 |
|
Double the normal rate |
| 4 |
|
35,000 |
| 5 |
|
Waived |
| 6 |
|
Requires approval from the EC Chairperson to waive the fee |
| Continuing Review | ||
| Certificate of Ethical Approval (CEA) Extension | ||
| Type of Fund | Fee (Baht) | |
| 1st CEA Extension | Waived | |
| 2nd CEA Extension | Waived | |
| 3rd CEA Extension (and subsequent): | Domestic Thai Fund under Mahidol University or Domestic Thai Non-Profit Organization | Waived |
| International Fund or Pharmaceutical Company or For-Profit Sponsor | 5,000/time | |
| Major Amendment | ||
| Type of Fund | Fee (Baht) | |
| 1st Amendment | Waived | |
| 2nd Amendment | Waived | |
| 3rd Amendment (and subsequent): | Domestic Thai Fund under Mahidol University or Domestic Thai Non-Profit Organization | 2,500/time |
| International Fund or Pharmaceutical Companies or For-Profit Sponsor | 5,000/time | |
Fees should be paid at the Finance Unit, Office of the Dean, 5th floor, The 60th Anniversary of His Majesty the King's Accession to the Throne Building, Faculty of Tropical Medicine, Mahidol University, before the submission of documents to the Committee for consideration. A copy of the payment receipt must be attached to the submitted documents.
3. After the Approval Has Been Granted, What Happens?
- The Certificate of Ethical Approval (CEA) is valid for one year
- An annual renewal is required each year until the end of the nominated study duration, or study completion, whichever comes first . Two months before the expiry date, the investigator should fill in form Progress Report Form and CEA extension request form (FTM ECF-008-06) ,and send it to the EC to request approval for extension. If the study is complete, a Notification of Study Closure (FTM ECF-010-04) is required.
- Investigator is required to submit the progress report annually after approval together with request for CEA extension and report according to EC requirement depending on risk involve by using Progress Report Form and CEA extension request form (FTM ECF-008-06) If an Investigator fails to submit a progress report by the scheduled review date, the EC will not extend the Certificate of Ethical Approval. Official notification letter will be provided to Investigators.
- If there are any changes to the research project (Change in the principal investigator or any co-investigator(s), Protocol, Participant information sheet, Informed consent form, Advertisement document, Questionnaire, etc,.) after it has been approved, an amendment must be submitted to, and approved by, the EC before the revised research project is implemented. A Request for Protocol Amendment Form (FTM ECF-023-04) is required for approval. Please indicate the version date on the footer of every page. Please note that a 5,000 Baht fee is charged in the case the project being funded by private- or foreign institution/company, and 2,500 Baht fee is charged in the case the project funded by government institution on the third amendment after the approval of the protocol. If it is amended in major revision (such as changing the information in synopsis, change or add main objective/ major issues ext.), the PI needs to submit it as a new research proposal.
-
The FTM EC will require that PI report all deaths that occur while the research participant is participating in a research study to the EC Chairperson. This must be accomplished in writing within five (5) working days of the death notification to PI.
The FTM EC will require that PI report all any unexpected situation affect serious to research project while the research participant is participating in a research study to the EC Chairperson (e.g., medication errors, unexpected complications, protocol violations). This must be accomplished in writing within five (5) working days of the event notification to PI, while the protocol deviations must be submitted in one (1) month.
For reporting local SAE which are fatal or life threatening the PI must report to EC immediately, no later than twenty four (24) hours after the PI becomes aware of the event while for the local SAE which is non-fatal or non lifethreatening the PI must report to EC immediately, no later than seven (7) calendar days after the PI becomes aware of the event.
For reporting any non-local Serious Adverse Reactions sponsor must report non-local serious adverse reaction including SUSARs to EC at least every six (6) months accompanied by a brief report highlighting the main point of concern. Other adverse reactions that may increase risks to subjects, the sponsor must report to EC as soon as possible but no later than fifteen (15) calendar days. Other type of reports, the sponsor must report to EC at least every year or periodically or on request.
In case of the SAEs occurring in different countries of a multicenter project, the Investigator can report to the FTM EC in one (1) month of the event notification to PI.
Investigators will submit the serious adverse event to the EC using the sponsor-required documentation. If such documentation is not available, the Investigator may use the SAE Report Form (FTM ECF-014-04).
For reporting local SUSARs which are fatal or life threatening sponsor must report to EC as soon as possible using CIOMS form, no later than seven (7) calendar days after the sponsor becomes aware of the event. If the initial report is incomplete, the sponsor must report to EC relevant follow-up information and complete report as soon as possible, within additional eight (8) calendar days. Sponsor must report any significant new information as a follow up report within fifteen (15) calendar days.
Local SUSARs which are non-fatal or non life threatening sponsor must report to EC as soon as possible using CIOMS form, no later than fifteen (15) calendar days after the sponsor becomes aware of the event. Further relevant follow-up information should be given as soon as possible.
The EC will require that PI report all Adverse Events related and not related to the study to the EC Chairperson. This must be accomplished in writing in one (1) year of the event notification to PI
PI will submit all safety information to the EC using the sponsor-required documentation.
- Once the study is complete or terminates under any other circumstance, the investigator must inform the EC of the study termination using the Notification of Study Closure (FTM ECF-010-04) and send it to the EC with the additional documentation indicated in the form.
4. Submit complete documentation to:
Secretariat OfficeEthics Committee of the Faculty of Tropical Medicine, Mahidol University
c/o Office of Research Services
4th Floor, The 60th Anniversary of His Majesty the King's Accession to the Throne Building
Faculty of Tropical Medicine, Mahidol University
420/6 Ratchawithi Road, Bangkok 10400, Thailand
Phone: 66 (0) 2354 9100-4 ext. 1349, 1525; or 66 (0) 2306 9126
Fax: 66 (0) 2306 9126
E-mail: ectropmed@gmail.com