Research Support
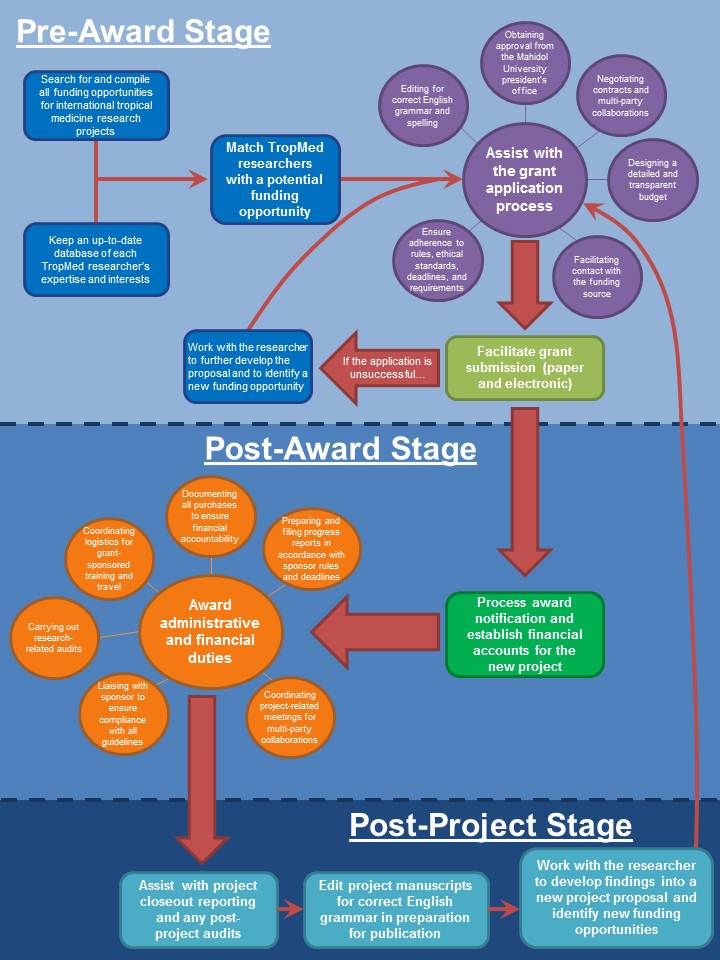
The Office of Research Services (ORS) coordinates and supports the research activities of the Faculty of Tropical Medicine (FTM) to achieve excellence in research and to retain its position as a leading research institution in Tropical Medicine.
The works in ORS is driven by Research Management Committee.
The Research Management Committee, overseeing all activities related to research. With respect to the Research Integrity & Compliance, all research performed within FTM systems are intended to be safeguarded. The experimental labs are regulated under the Biosafety & Infrastructure protocols. Consistent with this development, the Innovational Management is contributed to the pathway to its translational research.
The ORS is the central coordinator of all FTM's research activities. We provide guidance to staff on developing, submitting and managing research grants, as well as overseeing the regulatory standards related to research and facilitating true functionality of FTM's research.